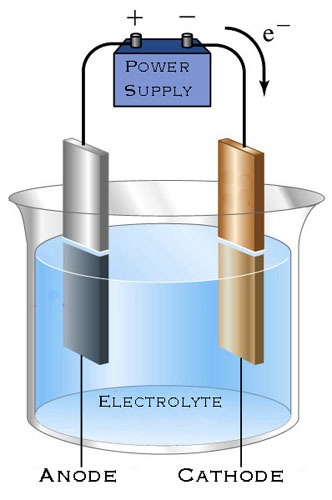
If you place two plates of the same metal in parallel - with no contact between them - into a solution made from a salt of the same metal and water to give a conducting liquid, then connect the plates to terminals of a direct current power supply or battery, the current will flow from one plate to the other through the solution (the electrolyte).
The electrolyte contains positive metal ions and negative sulfate ions. When the current flows, positive and negative ions of the electrolyte are attracted to the plate of opposite polarity. Positive metal ions are adhered or "attached" to the cathode (negative pole) and the negative sulfate ions are attracted to the bare areas of the anode (positive pole) and react with the metal surface oxidizing and eroding it*. The result of this process is a bite in the metal that is comparable with an acid etching; but with some very useful differences!
The plate you wish to etch is attached to the anode (+) and placed in the tank facing (in parallel with) another plate that is attached to the cathode (-), with a distance of 6 to 10 centimeters between them.
While positive metal ions are becoming solid metal at the cathode, an equivalent amount of metal is being extracted from the anode, thus the electrolyte keeps its original concentration.
The amount of sulfate in the solution does not change, and the electrolytic bath is reusable. The solution is not depleted with use. This balance and stability in the solution allows you to calculate bite-times more accurately than with acids.
By using the same concentration in the electrolytic solution, the same time, and same voltage, the printmaker is able to produce a consistent bite. If you have several plates of the same dimensions and etch identical areas on those plates using the same formula (i.e. consistent electrolyte concentration, time, voltage) you will produce identical results on all the plates.
This process does not release toxic gases such as those produced by etching zinc, copper and iron plates with nitric or hydrochloric acid. And, by taking some minor precautions when you introduce and remove plates from tanks or during the washing process for example, you can consider it a fairly harmless technique.
Electrolytic processes, using electricity, have the advantage of not producing waste, such as gas bubbles which with traditional acid etching can block the bite, nor does it produce sediment that can builds up on the plate and at the bottom of a tank as with other methods.
* For more information on the electrolytic process, see The basis of Electro-etching - a simplified explanation. By A Crujera and B Perkin